Failure of the glucotoxicity paradigm in type 2 diabetes
High blood glucose is the symptom of type 2 diabetes, not the disease
Tired of all the controversy, and still confident of the benefits of blood glucose lowering, the National Institutes for Health in the United States funded a huge, ambitious randomized controlled trial involving over 10,000 patients called the Action to Control Cardiac Risk in Diabetes (ACCORD) study to assess the benefits of intensive glucose control. After all, this was the standard diabetes advice of virtually every doctor in the world. Every medical school student had been indoctrinated to believe that this was the ‘best’ treatment approach.
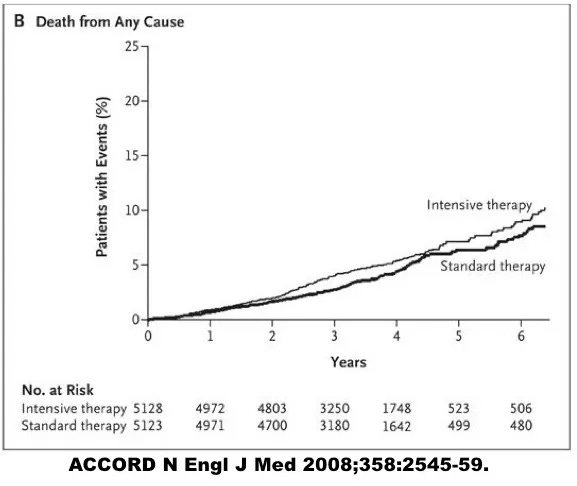
[caption id=”attachment_5396" align=”alignleft” width=”360"]
Increased mortality with intensive glucose control[/caption]
Intensive medical therapy certainly reduced the A1C from 7.5% to 6.5%, a large and meaningful reduction. Great, but that’s not the question we are interested in. Did this make any difference to health? It sure did. When the trial results broke in 2008, there was a media firestorm.
Why? Intensive treatment was killing people!
The safety committee forced a premature end to this trial. It was unethical to continue a treatment that was potentially lethal. Completely contrary to expectations, intensively treated patients were dying faster at a rate 22% higher than the standard treatment group despite, or perhaps because of the intervention. This equaled one extra death for every 95 patients treated. It could not be ethically allowed to continue, although this trial could not specify the reasons for the increased mortality.
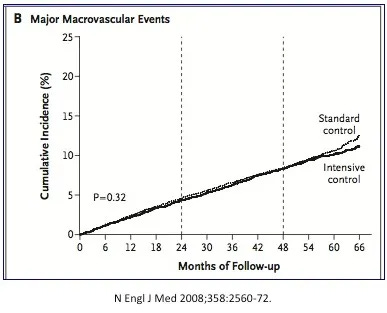
At the same time, the randomized double blind controlled ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial results were published. Once again, this blood glucose reducing strategy failed to deliver cardiovascular benefits, although at least there was no increase in mortality.
[caption id=”attachment_5398" align=”alignright” width=”387"]
ADVANCE study. No CV benefits to intensive glucose control[/caption]
Not all interventions were futile. The ADVANCE trial revealed that blood pressure lowering medications reduced cardiovascular disease, as expected. Certain medications did truly benefit patients, but those that reduced blood glucose did not.
Two further randomized controlled trials quickly followed to confirm these disappointing results. The Veterans Affair’s Diabetes Trial (VADT) found that medications to lower blood glucose produced no significant benefits to heart, kidney or eye disease.
The Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial treated pre-diabetic with early initiation of insulin, with the hope of reducing heart disease. Unfortunately, the answer is no. There was no reduction in heart disease, stroke, eye disease, or peripheral vascular disease. There were no measureable health benefits. Further experience with a new class of agents, the DPP4 inhibitors only confirmed the futility of blood glucose lowering as a therapeutic strategy.
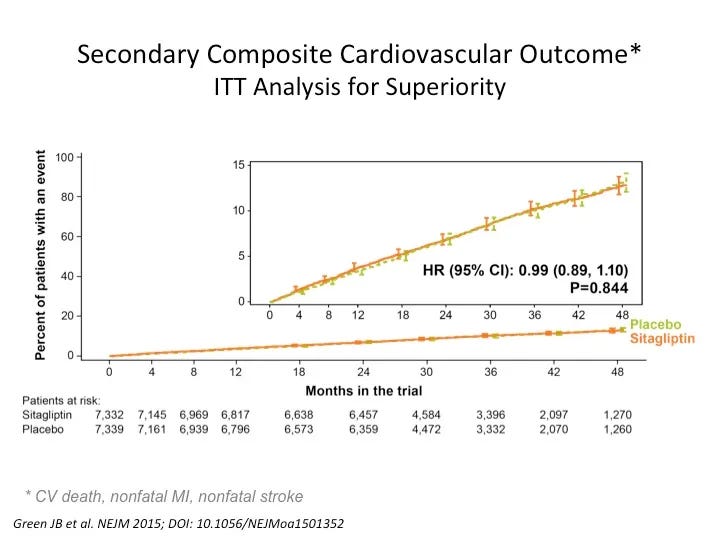
TECOS/ SAVIOR
In 2006, the FDA approved a new class of blood glucose lowering medication called the dipeptidyl peptidase 4 (DPP4) inhibitors. . Incretins are hormones released in the stomach, which increased the insulin secretion in response to food. The DPP4 inhibitors blocked the breakdown of the incretin hormones thus boosting levels. However, the insulin response was not sustained and thus, these drugs did not cause weight gain.
There were high hopes for the new DPP4 inhibitors. These drugs could lower blood glucose, with a low risk of hypoglycemia and no weight gain. The SAVIOR study was published in 2013 and the TECOS study was published in 2015, evaluating two new medications for the treatment of type 2 diabetes.
While there were no safety concerns, hopes were soon dashed that they would save lives. There were no protective effects, despite blood glucose lowering. Cardiovascular disease was not affected one way or the other.
The ACCORD, ADVANCE, and VADT trial all continued longer term follow up and published extended results (15,16, 18), but this yielded little new information. All trials agreed that intensive treatment did not save lives and had marginal benefits if any. Furthermore, there were adverse health consequences. Medications often increased weight gain and hypoglycemic reactions. Using more medications to lower blood glucose was obviously not beneficial.
The glucotoxicity paradigm, which formed the bedrock of medical treatment of type 2 diabetes was entirely, and irrevocably shattered. What was going on?
Inflammation
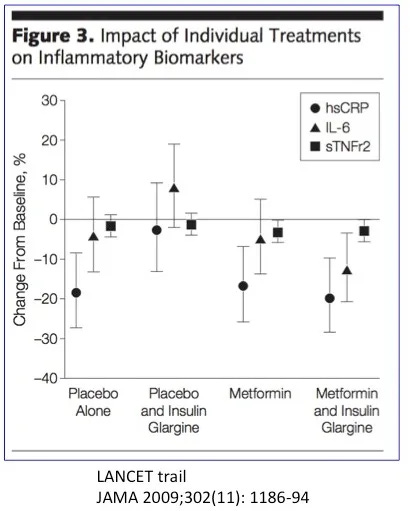
Atherosclerosis, the buildup of plaque in the arteries that contributes to heart disease and strokes is an inflammatory process, rather than simply cholesterol clogging up the artery like sludge in a pipe. This ‘hardening’ of the arteries is caused by injury to the lining of the blood vessel, which sets off the inflammatory response. Inflammatory mediators such as high sensitivity C-Reactive Protein (hsCRP), interleukin 6 (IL-6), and soluble tumor necrosis factor receptor 2 (sTNFr2) are measurable blood markers of this process and all independent predictors of cardiovascular disease.
Treatments that reduce blood vessel injury also reduce inflammation, a more easily measured marker. Does lowering blood sugars lower inflammation? Not so much. In the LANCET metformin trial, treatment reduced blood glucose but left inflammatory markers essentially unchanged. The insulin group raised hsCRP and IL-6, indicating more, not less inflammation. Yeah, that’s bad. Insulin is making things worse, not better. While insulin made blood sugars better, it made the diabetes worse. Drugs could not reduce the inflammation, and therefore could not prevent atherosclerosis, an inflammatory disease.
Likewise, the coronary artery calcification score, an indication of the burden of atherosclerotic plaque in the heart, is not correlated to measures of blood glucose control such as the A1C. But what was the problem?
Tradeoff
Standard medications for type 2 diabetes represent a tradeoff between glucotoxicity and insulin toxicity. Both insulin and SUs increase insulin to reduce hyperglycemia. The effect of the increased insulin becomes clinically obvious as weight gain, as hyperinsulinemia is the main driver of obesity. The price of better glucose control was higher insulin dosage, and there is no net benefit. These medications simply trade lower glucotoxicity for higher insulin toxicity.
Metformin and DPP4 medications use mechanisms other than raising insulin to lower blood glucose. But they do not lower insulin either. Once again, this manifests clinically with neither weight gain nor weight loss. Reducing glucotoxicity by itself produces minimal if any benefits. The hyperinsulinemia is the dominant feature of type 2 diabetes. Medications that do not lower the elevated insulin do no have benefits. Clinically, it becomes obvious that medications that lower blood glucose but do not lower body weight have no benefits.
Epidemiologic studies had shown a clear correlation between lower blood sugars and better health outcomes. Every 1% increase in the hemoglobin A1C was associated with an 18% increase in risk of cardiovascular events, 12–14% increase risk of death and a 37% increased risk of eye disease or kidney disease. But this was far from proof and made no distinction between medications and lifestyle measures.
Consider two type 2 diabetic patients with an identical A1C of 6.5%. One takes no medications and the other uses 200 units of insulin daily. Are these identical situations? Hardly. The first situation reflects mild diabetes while the other reflects severe diabetes requiring heavy doses of insulin. The cardiovascular risks are completely different and the use of medications does not reduce that risk.
The Hisayama Study compared A1C levels to risk of cardiovascular events. Importantly, this study differentiated between patients taking mediation versus those that did not. In those patients not taking medication, cardiovascular risk increased as the A1C increases. This is logical, since this reflects more severe type 2 diabetes.
What is telling though is the complete inability of the addition of diabetic medications to lower the risk of disease. This concurs with the evidence obtained through randomized controlled trials.
Recent research confirms the utter ineptitude of standard diabetic medications. Including all relevant trials up to March 2016, none of the drug classes considered, including metformin, SUs, TZDs and DPP4 inhibitors reduced cardiovascular disease or other complications despite the proven ability to lower blood glucose.
The results for insulin, when considered separately, came out even worse. Reviewing all available literature up to 2016, including twenty randomized controlled trials, researchers could only conclude that, “there is no significant evidence of long term efficacy of insulin on any clinical outcome in T2D (type 2 diabetes). However, there is a trend to clinically harmful adverse effects such as hypoglycaemia and weight gain.” In other words, insulin treatment carries no discernible benefits, but significant risks of adverse side effects. Insulin is “significantly more harmful than other active treatments”.
While the evidence is crystal clear, most diabetes guidelines have failed to reflect this new reality. Dr. Montori, of the Mayo Clinic reviewed published guidelines to find that 95% unequivocally endorsed benefit despite their non-existence.
The fact that insulin, SUs, metformin and DPP4 medications are proven to have no clinical impact on type 2 diabetes is of singular importance. Why would you take medications that have no benefits? Worse, why would you take medications that have no benefits and make you fat? These treatments should only be used when no other alternative is available for short-term reduction of blood glucose. But this situation never exists. As we shall see, there is always an available therapeutic lifestyle strategy. No, these medications are best described as “How NOT to treat type 2 diabetes”.



Does this mean that, as a non diabetic who has been low carb ( paleo but with dairy) for 8 yrs, I should not worry about my HbA1c creeping up to 5.7%?! I bought a keto-mojo meter and can see my waking glucose is around 6 mmol/L, going up to 6.8 during the morning (I thought the dawn phenomenon was supposed to be highest first thing?). It is unchanged after an hour dog walk hiking in the woods, but can go up to 8.1 after a heavy weightlifting session. It’s still 6 by the time I have my first meal at 2pm. Just prior to supper it’s around 5, goes up to 7 and then back to 5-6 after 2-3hrs. Ketones were 0.5 on normal 2 meal days but up to 1.1 with 24hr fast, up to 2-3 with 48hrs and 3-4 up to 72hr fast. Blood glucose steadily dropped with the 72hr fast, hitting a low of 3.2 during the 3rd day, but mostly being 4.2-4.4.
I am worried that having all this glucose sloshing around will damage my endothelia - but maybe that’s not the case according to these studies. I’m still concerned from an anti-cancer point of view. Is this physiological insulin resistance? I am overweight ( guessing my BMI is 26-27) but it’s all subcutaneous. I’ve put on weight due to overeating in an effort to increase my protein to try & build muscle. I think my increased dose of progesterone (as part of my HRT) has caused me to gain fat like when I was pregnant- surely this type of ‘physiological insulin resistance’ is not good for you?
In summary, so you’re not giving specific medical advice, does long term low carb/keto lead to physiological insulin resistance in everyone or only those people who overeat and gain weight?
Are higher than ‘desired’ glucose levels ok so long as the insulin is low? (My fasting insulin was 2 and glucose was 4 when taken at midday 5 yrs ago).
Does progesterone cause insulin resistance and is that undesirable unless you’re pregnant and needing to grow a baby?
Does overeating protein lead to raised glucose levels- and is that just on a per meal basis, or is it a longer lasting effect over the following days?
Thanks!
Thank you AGAIN.